
Diatoms - Nature’s Jewels viewed with a Microscope
Posted by Motic America on
Introduction
Diatoms are unicellular algae (Division Chrysophyta, Class Bacillariophyceae). Diatoms are microscopic in size, live in water, soil and moist environments, and exhibit highly ornamented glass houses made of silica with two parts that fit together like a Petri dish. Diatoms play an important role in producing about 20% of the earths’ oxygen and form an important part of the food chain feeding other aquatic life. They can be observed with a compound light microscope (see Motic’s microscope selection). Diatoms are found world-wide and were first discovered and shown in a drawing by an Englishman, Charles King in 1703 - Tabellaria sp. (D. G. Mann, 2020). Since then diatoms have been studied by both amateur microscopists and researchers for several hundred years.

Diatoms contain photosynthetic chemicals chlorophyll a, c, along with accessory pigments ß-carotene and fucoxanthin. Some diatom species also have a raphe, a longitudinal structure which divides symmetrical diatoms in the middle. Diatoms with a raphe can move and extrude mucilage and their trails can be stained using Stains-all (L. Chen et al. 2019). Studies also suggest movement of diatoms appears to involve actin, myosin and proteoglycans (C. Nicole et al., 1999, J.L. Lind et al., 1997), but the mechanism of movement is still not fully understood. Diatoms exhibit positive photo-taxis (move toward the light) and some species can move without coupling to a substrate (T. Harbich, 2021).
Diatoms reproduce by cell division and sexually via gametes. Diatoms get smaller as they divide and then they form an auxospore through a sexual process by which the diatom returns to full size. Diatoms are classified as algae, technically they are neither plant nor animal but are protists -any eukaryotic organism whose cells contain a cell nucleus that is not an animal, plant, or fungus.
Macroscopically, diatoms often appear as a slimy bio-film on rocks, and cement walls with running water. This scum when viewed with a bright-field light microscope shows their beauty. Diatoms are often cleaned and prepared for mounting on a microscope slide by burning them, washing them in hydrogen peroxide, acid or bleach to remove their intracellular components leaving only their clear silica (glass) shells. The silica shells can survive for millions of years forming large diatom deposits which are mined (e.g. Red Lake Earth deposit in British Columbia).
Researchers estimate there are at least 10,000 species of Diatoms world-wide. Their taxonomy is primarily based on their shell structure and there are two main groups of diatoms: centric and pennate diatoms. (For more information on diatom biology and keys to their identification see J. P. Kociolek et al., 2015, Diatoms.org, L. Bahls et al., 2018, E.J Cox 1996, W.C. Vinyard, 1979, S. Stidolph et al. 2012, and Spaulding et al. 2021.)
Once live diatoms are cleaned and mounted on slides they can be drawn or photographed showing their ornate details. Diatoms are often mounted in a high refractive index medium (e.g. Napthrax) which results in higher contrast making diatoms more visible. It is important to use thin No. 1-1.5 cover glass (O.017 mm thick) to cover the diatoms for taking the sharpest photographs. Some diatomists mount their diatoms on the coverslip first then mount the coverslip on a slide.
Identification of diatoms to species often requires specialists. Attempts to automatically identify diatoms using software and artificial intelligence are still under development. Some amateur diatom collectors arrange diatoms on microscope slides to form art-like patterns. This process can take a long time to make a single slide. Prepared slides of diatoms are also used to test the optical resolution of light microscopes e.g. Pleurosigma angulatum (J.B. Anderson, 1990). Diatoms are also used in forensic science in a variety of ways, the most frequent being the diagnosis of death by drowning (Forensics of Diatoms). To study diatoms at high magnifications researchers use transmission and scanning electron microscopes.
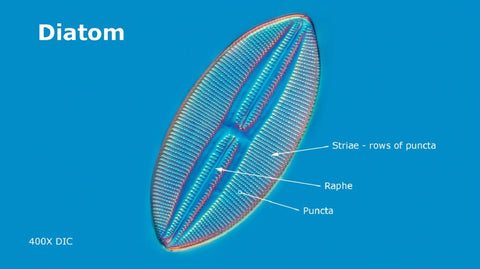

Diatoms generally range in size from about 20 to 300 microns though some can be a millimeter or larger. Many diatoms have puncta or small holes in their silica shells. Pores allow diatoms to obtain some nutrients from the environment and secrete extracellular polysaccharides.












Photographing Diatoms and Focus stacking
When viewing diatoms with a microscope the depth of field decreases when using higher magnification objectives. One way to increase the depth of field is to take a series of photographs at different focus levels by moving the fine focus between each picture frame. The images are then loaded into layers in a software program, aligned and blended. You can use as few as 2 images or as many as 50 or more. Below are images of a diatom that has been focus stacked using Photoshop, Helicon Focus, and Zerene software (there are other programs).


Diatomaceous Earth – Fossilized Diatoms
Diatomaceous earth is made up of diatom fossils consisting of about 80-90% silica with some alumina (2-4%) and 0.5 to 2% iron (Wikipedia). Fossilized diatoms are available from retailers online and some stores. Diatomaceous earth is used as: a filtration aid, mild abrasive, filler, support for chemical catalysts, in litter boxes, as a stabilizer in dynamite, thermal insulation, in potted plants and to absorb fluids. In addition it is used as an insecticide and kills: ants, bedbugs, silver fish, flies, flour beetles, and spider mites. It acts by lacerating the digest tract and absorbing oils and fats from the insect cuticle (Health Canada). It also kills beneficial insects such as bees so be careful if using it in your garden.
Food grade diatomaceous earth is used as an additive for cattle, but it should not be more than 2% of the animal’s total diet. It is not recommended for consumption by humans in spite of what some websites indicate (see Health Canada web site). Diatomaceous earth is a respiratory irritant and although it has low toxicity workers that use diatomaceous earth require proper protective clothing, gloves, masks, and eyewear. Workers should not be exposed to diatomaceous earth for more than 4 hours per day. Diatomaceous earth must be kept out of reach of children and pets.
In Canada diatomaceous earth is mined. The deposits can be from either freshwater, or ocean water. In British Columbia it is mined in Red Earth north of Kamloops. In agriculture use, diatomaceous earth is used as an anti-caking product. In a silo of wet grain, corn and other types of feed will often stick together creating clumps. Diatomaceous earth helps to dry out the feed and keep it from sticking. Diatomaceous earth is being researched for use in drug delivery and in various forms of nano-technology and sensing devices (M. Villani et. al. 2019).
Natural diatomaceous earth is non-calcined (not exposed to high heat) is not considered harmful and can be ingested by animals in small amounts. Diatomaceous earth is also heated and crushed into a powder, however the remnants of diatom shells may still be seen with a microscope. I would not recommend that people ingest it especially after you see what it looks like when viewed with a microscope (see pictures below) since the diatom fragments are made up of sharp pieces of glass.


Summary
Diatoms are unicellular algae (protists) with silica houses and are found in freshwater, salt water, moist soil, and in mosses. There are two basic forms: Centric and Pennate diatoms. The pennate diatoms are further divided into those with a raphe and those without. Diatoms with a raphe are able to move on a substrate. Diatoms reproduce asexually and sexually, and form about 20% of the world’s oxygen and convert carbon dioxide to oxygen and sugar. Diatom taxonomy is based primarily on their frustule morphology and more recently on DNA analysis. The study of diatoms requires a light microscope to observe them. Fossil diatoms create Diatomaceous earth which is mined and used in polishes, filters, fillers and as an insecticide. Since diatomaceous earth contains diatom fragments which kills insects by lacerating their digest tract. In the future diatoms are being investigated as a biofuel source, as sensors in nanotechnology, and for drug delivery. For those interested in studying diatoms Motic offers a variety of light microscopes and cameras that can be used to investigate and photograph them.
References
- D.G. Mann (2020) Introduction to Diatoms discovery: Royal Botanic Garden Edinburgh https://websites.rbge.org.uk/algae/diatoms_introduction1.html
- Author of the anonymous scientific communication was discovered by Dolan, J. (2019). Unmasking "The Eldest Son of The Father of Protozoology": Charles King". Protist 170: 374-384.
- L. Chen, D. Weng, C. Du, J. Wang, and S. Cao. (2019) Contribution of frustules and mucilage trails to the mobility of diatom Navicula sp. Scientific Reports 9: 7342. https://www.nature.com/articles/s41598-019-43663-z
- N. C. Poulsen, I. Spector, T. P. Spurck, T. F. Schultz, R. Wetherbee (1999) Diatom gliding is the result of an actin-myosin motility system. Cytoskeleton: 44: 23-33. https://doi.org/10.1002/(SICI)1097-0169(199909)44:1<23::AID-CM2>3.0.CO;2-D
- J. L. Lind, K. Heimann, E. A. Miller, C. van Vliet, N. J. Hoogenraad, R. Wetherbee (1997) Substratum adhesion and gliding in a diatom are mediated by extracellular proteoglycans 1997 Planta 203(2):213-21. doi: 10.1007/s004250050184.
- T. Harbich (2021) Some Observations of Movements of Pennate Diatoms in Cultures and Their Possible Inter+pretation in S.Cohn et al. (eds) Diatom Gliding Motility, pg 1-32. Scrivener Publishing LLC.
- J.P. Kociolek, E.C. Theriot, D.M. Williams, M. Julius, E. F. Stoermer and J. C. Kingston (2015) Chapter 15 Centric and Araphid Diatoms in Freshwater Algae of North America. Elsevier.
- J.P. Kociolek, S.A., Spaulding, and R.L. Rowe (2015) Bacillariphyceae: Chapter 16 The Raphid Diatoms in in Freshwater Algae of North America. Elsevier.
- Spaulding et al. (2021). Diatoms.org: Diatoms of North America supporting taxonomists, connecting communities. Diatom Research 36(4): 291-304. doi:10.1080/0269249X.2021.2006790 Visit web site: https://diatoms.org/
- L. Bahls, B. Boynton, B. Johnstone (2018) Atlas of diatoms (Bacillariophyta) from diverse habitats in remote regions of Western Canada. PhytoKeys 105: 1-186. https://phytokeys.pensoft.net/browse_journal_articles.php?form_name=filter_articles&sortby=0&journal_id=3&search_hidden=Diatoms+of+Western+Canada&search_in_=0§ion_type%5B%5D=83
- E. J. Cox (1996) Identification of Freshwater Diatoms from Live Material. Chapman and Hall – book is out of print, but PDF versions can be found online.
- S. R. Stidolph, F.A.S Sterrenburg, K.E.L. Smith, and A. Kraberg, ( 2012) Diatom Atlas: U.S. Geological Survey Open-File Report 2012–1163, available online at http://pubs.usgs.gov/of/2012/1163/. 204 pages of photos to aid identification of North American diatoms.
- W.C. Vinyard (1979) Diatoms of North America. Mad River Press. California 120 pages with illustrations of the most common diatoms.
- J. B. Anderson (1990) The Measurement of Three Light Microscope Test Diatoms by Scanning Electron Microscopy. Proceedings RMS: 25: 195-203. http://www.microscopist.co.uk/wp-content/uploads/2017/04/Diatom-test-paper.pdf
- C. Sanchez, G. Christobal, G. Bueno, S. Blanco, M. Borrego-Ramos, A. Olenici, A. Pedraza, J. Rusiz-Santaguiteria (2017) Optical illumination in microscopy: a quantitative evaluation. Micron prepint. https://core.ac.uk/download/pdf/132611224.pdf
- Forensic of Diatoms in Drowning: Extraction and Procedure https://forensicreader.com/forensic-analysis-of-diatoms/
- Y. Maeda, D. Nojima, T. Yoshino and T. Tanaka (2017) Structure and properties of oil bodies in diatoms. Phil. Trans. R. Soc. B 372. https://www.researchgate.net/publication/318500447_Structure_and_properties_of_oil_bodies_in_diatoms.pdf
- R. Berdan (2017) Focus Stacking comparing Photoshop, Helicon Focus and Zerene. https://www.canadiannaturephotographer.com/rberdan_focus_stacking.html
- Wikipedia (2022) Diatomaceous Earth https://en.wikipedia.org/wiki/Diatomaceous_earth
- Health Canada regulations regarding the use of Diatomaceous earth in food https://www.canada.ca/en/health-canada/services/consumer-product-safety/pesticides-pest-management/public/consultations/proposed-registration-decisions/2020/silicon-dioxide-dx13/document.html
- M. Villani et al (2019) Transforming diatomaceous earth into sensing devices by surface modification with gold nanoparticles. Micro and Nano Engineering 2:29-34. https://www.sciencedirect.com/science/article/pii/S2590007218300169
- Red Lake Diatomaceous Earth in British Columbia, north of Kamloops - https://cmscontent.nrs.gov.bc.ca/geoscience/PublicationCatalogue/Paper/BCGS_P2004-02-50_Aylen.pdf Website for Diatomaceous earth: https://www.redlakeearth.com/
- Photo-taxis of diatoms and desmids showing how they respond to light of different colours and move – movies. https://av.tib.eu/media/10906
- Diatomaceous earth video: Youtube: https://www.youtube.com/watch?v=DUfYbZkkFJ0
Related Products
| Models | |||
| Features |
The BA310E LED Phase package contains everything in the BA310E LED along with phase accessories including a turret condenser, phase objectives and phase centering telescope. |
The Panthera C2 phase package is a future-orientated solution, which includes everything in Panthera C2 and all the phase accessories required for phase contrast applications. |
Our Moticam A Series cameras are designed with microscopy beginners, teaching environments, hobbyists, and small labs in mind. Camera resolution ranging from 1MP - 16MP. |
Want to know which microscopes fit you best? Fill in the form below and our specialists are glad to help!





